- 产品特性
- 相关资料
- Q&A
- 参考文献
BAMBANKER®
无血清细胞冻存液
|
BAMBANKER® 是一种无血清细胞冻存液。可在 -80℃ 长期保存细胞(肿瘤细胞和常规细胞)。 |
 |
◆产品特性
● 即用型细胞冻存液
● 无需分步降温,直接使用
● 无需稀释
● 无需程序降温盒
● -80℃ 长期保存
● 无血清
◆无血清冻存液的优点
● 与含血清类型相比,批次间的成分组成差异小,可保持稳定的品质。
● 不含血清,因此没有因动物源的未知成分和感染物质所产生的影响与风险。
● 可对无血清驯化细胞进行冷冻,节省再驯化步骤。
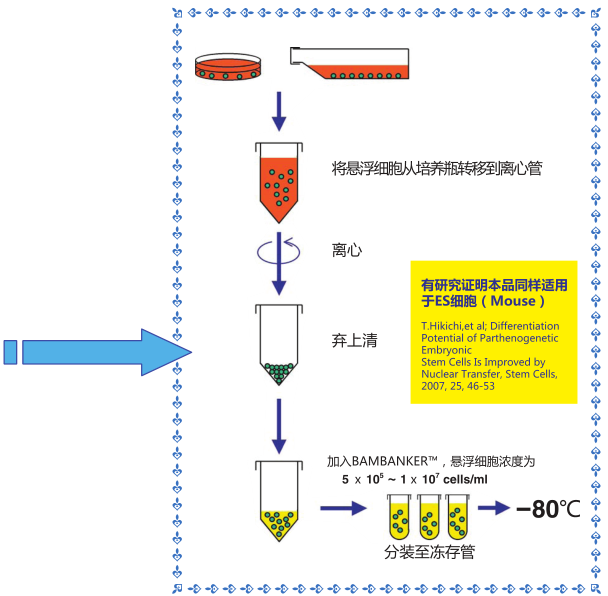
◆操作流程
1)收集生长对数期*的细胞(5×105-1×107个细胞)
2)用1mL 该细胞冻存液悬浮细胞,置于冻存管中,不需预冷,直接 -80℃ 冷冻保存,也可在-80℃冻存 12 小时后可
转移至液氮中保存。
3)用恒温箱或者水浴锅快速复苏细胞
*冷冻细胞必须处于生长对数期
◆无菌检测**
内毒素:生色底物法
支原体:荧光抗体法
真菌和细菌:依据日本药典
(**:可索取检验证书)
BAMBANKER® Direct
BAMBANKER Direct 是“无血清型”细胞冻存液。
◆BAMBANKER® Direct无需离心收集细胞
 ① 不需冻存前预处理,操作简便
① 不需冻存前预处理,操作简便
② 不需稀释,直接使用
③ 无需分步降温,直接使用
④ 可快速、长期冻存细胞(-80℃或液氮)
⑤ 不含血清
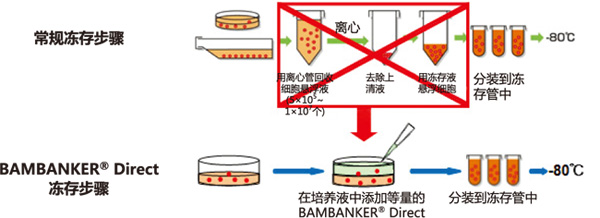
◆BAMBANKER® Direct冻存步骤VS常规冻存步骤
使用本产品无需经过离心等复杂步骤,只需往培养基内添加与培养液等量的 BAMBANKER Direct,再分装到冻存管,置于-80℃ 便可冻存细胞。
◆应用
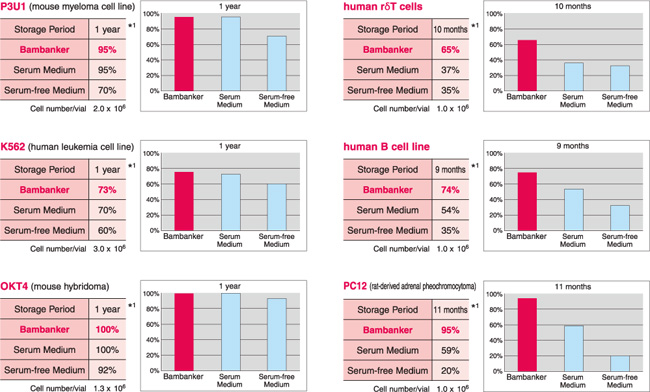
【BAMBANKER使用例】
| 细胞名称 | 保存时间 |
生存率 | ||
| BAMBANKER | 公司A(含血清) |
公司A(不含血清) |
||
|
P3U1 (小鼠骨髓瘤细胞系) |
12个月 |
95% | 95% | 70% |
|
K562 (人白血病细胞系) |
12个月 |
73% | 70% | 60% |
| 人体胃黏膜上皮细胞 | 10个月 |
100% | 62% | 56% |
|
human γδT cells (人γδT细胞) |
10个月 |
65% | 37% | 35% |
|
Daudi (人 B细胞系) |
12个月 |
100% | 100% | 92% |
|
PC12 (大鼠源肾上腺嗜铬细胞瘤) |
11个月 |
95% | 59% | 20% |
|
human B cell line (人B细胞系) |
9个月 |
74% | 54% | 35% |
|
OKT4 (小鼠杂交瘤细胞) |
12个月 |
100% | 100% | 92% |
|
B细胞系 (猴) |
10个月 | 56% | 40% | 18% |
【低温冻存实验证明以下细胞保存完好】
|
3T3‐L1(小鼠前脂肪细胞系) |
A431(人扁平上皮癌细胞系) |
BAEC(牛主动脉血管内皮细胞系) |
|
Balb/3T3(小鼠成纤维细胞系) |
C2C12(小鼠骨骼肌细胞系) |
Daudi(人B细胞系) |
|
ECV304(人脐静脉内皮细胞系) |
H295R(肾上腺皮质细胞) |
HEK293(人胚胎肾细胞系) |
|
HEK293T(人胚胎肾细胞系) |
HeLa(人子宫颈癌细胞系) |
HeLa S3(人子宫颈癌细胞系) |
|
HepG2(人肝癌细胞系) |
HFF(人正常成纤维细胞系) |
Huh7(人肝癌细胞系) |
|
Jurkat(人白血病T细胞系) |
K562(人慢性骨髓性白血病细胞系) |
KATOIII (人胃癌上皮细胞系) |
|
KLM‐1(人胰腺癌细胞系) |
MDCK(犬肾小管上皮细胞系) |
MEF(小鼠胚胎成纤维细胞) |
|
NIH3T3(小鼠胚胎皮肤细胞) |
OKT4(小鼠杂交瘤细胞) |
OP9(小鼠骨髓基质细胞) |
|
P3U1(小鼠骨髓瘤细胞系) |
PANC‐1(人胰腺癌细胞系) |
PC12(大鼠源肾上腺嗜铬细胞瘤) |
|
RPE(人视网膜上皮细胞系) |
SNL(小鼠胚胎成纤维细胞) |
TSU‐Pr1(人前列腺癌细胞系) |
|
Vero(非洲绿猴肾细胞系) |
human γδT cells (人γδT细胞) |
human B cell line (人B细胞系) |
| HDF(人皮肤成纤维细胞) | HCC20(人乳腺原发性导管癌细胞) | BMMCs(人骨髓单核细胞系) |
| BMMCs(猪骨髓单核细胞系) | BMSCs(马骨髓间充质干细胞系) | C1(人成纤维细胞系) |
| CEF(牛胚胎成纤维细胞) | CHO-K1(中国仓鼠卵巢细胞系) | DPCs(大鼠牙髓细胞) |
| DPCs(人牙髓细胞) | ESCs(人胚胎干细胞) | EVT(人绒毛外滋养层细胞) |
| GH3(大鼠垂体瘤细胞) | Gli36(胶质瘤细胞系) |
h1(人类胚胎干细胞) |
| h9(人类胚胎干细胞) | HN4(人口腔上皮细胞系) |
HS-RMS-2 (多形性横纹肌肉瘤细胞系) |
| IPS(人诱导性多能干细胞) |
LNCaP clone FGC (人前列腺癌细胞) |
MCF 10A(人正常乳腺细胞) |
|
MEF-BL/6-1 (小鼠胚胎成纤维细胞) |
MNCs(人单核细胞) | MSCs(大鼠间充质干细胞系) |
| PBMCs(人外周血单个核细胞) | PDL(人牙周膜细胞) | pES(大鼠孤雌胚胎干细胞系) |
| Sf9(草地贪夜蛾细胞系) | U251(胶质瘤细胞系) | U87(胶质瘤细胞系) |
| VT(人绒毛膜滋养层细胞) | 癌症干细胞 | 大鼠肝细胞 |
|
猴B细胞系 |
人外周血活化淋巴细胞 |
永生化人肌肉细胞 |
|
小鼠脾脏活化淋巴细胞 |
小鼠ES细胞系 |
人胃上皮细胞 |
| 大鼠神经祖细胞 | 大鼠脂肪细胞 | 狗肿瘤细胞 |
| 胶质瘤细胞系 | 牛脂肪细胞 | 牛子宫内膜上皮细胞 |
| 人扁桃体细胞 | 人肝细胞 | 人骨髓CD34+细胞 |
| 人巨噬细胞 | 人淋巴细胞 | 人输卵管上皮细胞 |
| 人胎儿卵巢成纤维细胞 | 人胎儿卵巢体细胞 | 人自然杀伤细胞 |
| 神经祖细胞 | 小鼠颅骨成骨细胞 | 心肌祖细胞 |
| 猪成纤维细胞 |
ES细胞(小鼠)使用实例
T.Hikichi,et al; Differentiation Potential of Parthenogenetic Embryonic Stem Cells Is Improved by Nuclear Transfer, Stem Cells, 2007, 25, 46-53
更多相关资料请点击文字:
BAMBANKER® 与自制冻存液的冻存效果比较
※ 本页面产品仅供研究用。研究以外不可使用。
Bambanker® 与其他相关产品的比较
细胞冻存效果验证
细胞冻存液类型
1.Bambanker®
2.Medium with serum (含血清,A公司)
3.Serum-free Medium (无血清,A公司)
实验结果
*1:细胞-80℃的保存时间
相关PDF
|
BAMBANKER® 无血清细胞冻存液 |
Wako BAMBANKER冻存液(新手册) 细胞种类列举 |
BAMBANKER细胞冻存液 –冻存解冻步骤说明 (终端).pdf |
|
1. |
Q:为什么我使用了 BAMBANKER® 来保存细胞,但是存活率依然不高? A:请冻存前确保细胞处于生长对数期,并且冻存时细胞数目控制在 5×105~1×107/mL 冻存液。 |
|
2. |
Q:我们实验室已经有固定的冻存程序了,换了你们的 BAMBANKER® 可以还继续用原来程序降温的方法冻存吗? A:虽然本产品可以无需程序降温冻存细胞,但如果通过程序降温盒等适当控制了温度下降的速度,效果更佳。 |
|
3. |
Q:哪些细胞株(系)适合使用BAMBANKER® 来进行细胞冷冻保存? A:几乎所有细胞株(系)都可以使用 BAMBANKER® 进行冻存。对于较为宝贵的 ES/iPS 细胞的保存尤其适用。官网上所列举的细胞系均已经过测试验证。 但也并不排除可能有某些细胞株是不适合使用 BAMBANER® 来进行冻存的,用户在没有确认是否可使用时,建议在进行正式细胞冻存之前先进行预实验。 |
|
4. |
Q:无血清冻存液相比传统含血清冻存液有什么优势? A:无血清冻存液因不含有动物血清,质量更稳定,批间差小;同时未知生物成分或感染性物质污染细胞的几率也极低,尤其对于 ES/iPS 细胞等有可能用于再生医疗的细胞安全得以严格保障;可以直接冻存无血清培养的细胞,免去无血清再驯化的步骤;另外 BAMBANKER® 无血清细胞冻存液不需要像传统的血清冻存液需要程序降温,减少了用户的繁琐操作,节省了时间。 |
|
5. |
Q:BAMBANKER® 在冷冻保存细胞的过程中起什么作用? A:BAMBANKER® 无血清细胞冻存液使用了 DMSO 等作为保护剂,在冻存细胞时能以1℃/min 左右的温度下降而逐渐冻结,在此过程中,细胞内的水分子被置换成冻结保护剂,抑制胞内和细胞周边的冰晶的形成,防止细胞膜和细胞器结构损伤,防止蛋白变质。 |
|
6. |
Q:未使用的BAMBANKER® 无血清细胞冻存液应该如何保存? A:2-10℃ 避光保存。开封后尽快使用。请注意保质期为自生产日期起 24 个月。 |
|
7. |
Q:BAMBANKER® 能否使用于医疗领域? A:BAMBANKER® 仅供科研使用,不能使用于人体或医疗领域。 |
BAMBANKER™参考文献
|
[1] |
Zhang C., Seo J., Nakamura T. (2018) Cellular Approaches in Investigating Argonaute2-Dependent RNA Silencing. In: Okamura K., Nakanishi K. (eds) Argonaute Proteins. Methods in Molecular Biology, vol 1680. Humana Press, New York, NY. |
|
[2] |
Sharma, A., M¨ucke, M., & Seidman, C. E. (2018). Human induced pluripotent stem cell production and expansion from blood using a non-integrating viral reprogramming vector. Current Protocols in Molecular Biology,122, e58. doi: 10.1002/cpmb.58. |
|
[3] |
Souta Motoike, Mikihito Kajiya, Nao Komatsu, et al. Cryopreserved clumps of mesenchymal stem cell/extracellular matrix complexes retain osteogenic capacity and induce bone regeneration. Stem Cell Res Ther. 2018; 9: 73. Published online 2018 Mar 21. doi: 10.1186/s13287-018-0826-0. |
|
[4] |
Konuma T1, Kohara C1, Watanabe E2, et al. Monocyte subsets and their phenotypes during treatment with BCR-ABL1 tyrosine kinase inhibitors for Philadelphia chromosome-positive leukemia. Hematol Oncol. 2018 Apr;36(2):451-456. doi: 10.1002/hon.2497. Epub 2018 Feb 12. |
|
[5] |
Srijaya Thekkeparambil Chandrabose, Sandhya Sriram, et al. Amenable epigenetic traits of dental pulp stem cells underlie high capability of xeno-free episomal reprogramming. Stem Cell Research & Therapy 2018 9:68. |
|
[6] |
Evans, Michael A. et al. "Macrophage-Mediated Delivery of Light Activated Nitric Oxide Prodrugs with Spatial, Temporal and Concentration Control." Chemical Science (2018): n. pag. Web. doi:10.1039/C8SC00015H. |
|
[7] |
Jauregui, C.; Yoganarasimha, S.; Madurantakam, P. Mesenchymal Stem Cells Derived from Healthy and Diseased Human Gingiva Support Osteogenesis on Electrospun Polycaprolactone Scaffolds. Bioengineering 2018, 5, 8. |
|
[8] |
Khamaikawin, Wannisa et al. Modeling Anti-HIV-1 HSPC-Based Gene Therapy in Humanized Mice Previously Infected with HIV-1. Molecular Therapy – Methods & Clinical Development , Volume 9,23-32. |
|
[9] |
Masako Okumura, Toyoaki Natsume, Masato T Kanemaki, Tomomi Kiyomitsu. Optogenetic reconstitution reveals that Dynein-Dynactin-NuMA clusters generate cortical spindle-pulling forces as a multi-arm ensemble. bioRxiv 277202; doi: https://doi.org/10.1101/277202 |
|
[10] |
https://labchem.wako-chem.co.jp/journal/docs/proup10.pdf<链接> |
|
[11] |
Ince T A, Aster J C. In vitro culture conditions for T-cell acute lymphoblastic leukemia/lymphoma: U.S. Patent 9,683,217[P]. 2017-6-20. |
|
[12] |
Morris C D, Azadnia P, de Val N, et al. Differential Antibody Responses to Conserved HIV-1 Neutralizing Epitopes in the Context of Multivalent Scaffolds and Native-Like gp140 Trimers[J]. mBio, 2017, 8(1): e00036-17.<链接> |
|
[13] |
Lee K, Saetern O C, Nguyen A, et al. Derivation of Leptomeninges Explant Cultures from Postmortem Human Brain Donors[J]. JoVE (Journal of Visualized Experiments), 2017 (119): e55045-e55045.<链接> |
|
[14] |
Buenrostro J D, Corces R, Wu B, et al. Single-cell epigenomics maps the continuous regulatory landscape of human hematopoietic differentiation[J]. bioRxiv, 2017: 109843.<链接> |
|
[15] |
Edmonds R E, Garvican E R, Smith R K W, et al. Influence of commonly used pharmaceutical agents on equine bone marrow‐derived mesenchymal stem cell viability[J]. Equine veterinary journal, 2017, 49(3): 352-357.<链接> |
|
[16] |
Jitraruch S, Dhawan A, Hughes R D, et al. Cryopreservation of Hepatocyte Microbeads for Clinical Transplantation[J]. Cell transplantation, 2017.<链接> |
|
[17] |
Usarek E, Barańczyk-Kuźma A, Kaźmierczak B, et al. Validation of qPCR reference genes in lymphocytes from patients with amyotrophic lateral sclerosis[J]. PloS one, 2017, 12(3): e0174317.<链接> |
|
[18] |
Gagnon E, Connolly A, Dobbins J, et al. Studying Dynamic Plasma Membrane Binding of TCR-CD3 Chains During Immunological Synapse Formation Using Donor-Quenching FRET and FLIM-FRET[J]. The Immune Synapse: Methods and Protocols, 2017: 259-289.<链接> |
|
[19] |
Foster K, Chaddock J, Penn C, et al. Non-cytotoxic protein conjugates: U.S. Patent 9,474,807[P]. 2016-10-25. |
|
[20] |
Araki N, Iida M, Machida K. Bioassay method for detecting physiologically active substance: U.S. Patent 9,316,588[P]. 2016-4-19. |
|
[21] |
Sazinsky S, Michaelson J S, Sathyanarayanan S, et al. Antibodies to icos: U.S. Patent Application 15/076,867[P]. 2016-3-22 |
|
[22] |
李凯. Studies on Innate Immune Activation by HBV Infection and Its Sensing Mechanism in Hepatocytes[J]. 2016. |
|
[23] |
Ip L R H. Effect of INPP4B loss on DNA repair and treatment strategies in ovarian cancer[D]. UCL (University College London), 2016. |
|
[24] |
Thakkar A. Novel hormonal combination therapy for triple negative breast cancer[D]. University of Miami, 2016. |
|
[25] |
Pakdaman Y. In-vitro characterization of STUB1 mutations in recessively inherited spinocerebellar ataxia-16[D]. The University of Bergen, 2016. |
|
[26] |
Caxaria S. Induced pluripotent stem cells (iPSCs) for research and therapy: induction of hepatic differentiation in iPSCs and evaluation of their quality as a model of in vivo development in the context of coagulation[D]. UCL (University College London), 2016.<链接> |
|
[27] |
Bayne R A, Donnachie D J, Kinnell H L, et al. BMP signalling in human fetal ovary somatic cells is modulated in a gene-specific fashion by GREM1 and GREM2[J]. MHR: Basic science of reproductive medicine, 2016, 22(9): 622-633.<链接> |
|
[28] |
Friedrich D. HIF-1 [alpha] Drives Fungal Immunity in Human Macrophages[D]. Universität zu Lübeck, 2016.<链接> |
|
[29] |
Yasuda M, Kawabata J, Akieda-Asai S, et al. Guanylyl cyclase C and guanylin reduce fat droplet accumulation in cattle mesenteric adipose tissue[J]. The Journal of Veterinary Science, 2016.<链接> |
|
[30] |
Campa M J, Moody M A, Zhang R, et al. Interrogation of individual intratumoral B lymphocytes from lung cancer patients for molecular target discovery[J]. Cancer Immunology, Immunotherapy, 2016, 65(2): 171-180.<链接> |
|
[31] |
Kobayashi T, Yagi Y, Nakamura T. Development of Genome Engineering Tools from Plant-Specific PPR Proteins Using Animal Cultured Cells[J]. Chromosome and Genomic Engineering in Plants: Methods and Protocols, 2016: 147-155.<链接> |
|
[32] |
Shikata H, Kaku M, Kojima S I, et al. The effect of magnetic field during freezing and thawing of rat bone marrow-derived mesenchymal stem cells[J]. Cryobiology, 2016, 73(1): 15-19.<链接> |
|
[33] |
Hirakawa M, Matos T, Liu H, et al. Low-dose IL-2 selectively activates subsets of CD4+ Tregs and NK cells[J]. JCI insight, 2016, 1(18).<链接> |
|
[34] |
Durruthy-Durruthy J, Sebastiano V, Wossidlo M, et al. The primate-specific noncoding RNA HPAT5 regulates pluripotency during human preimplantation development and nuclear reprogramming[J]. Nature genetics, 2016, 48(1): 44-52.<链接> |
|
[35] |
Caxaria S, Arthold S, Nathwani A C, et al. Generation of integration-free patient specific iPS cells using episomal plasmids under feeder free conditions[J]. Patient-Specific Induced Pluripotent Stem Cell Models: Generation and Characterization, 2016: 355-366.<链接> |
|
[36] |
Nonomura Y, Otsuka A, Nakashima C, et al. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients[J]. Oncoimmunology, 2016, 5(12): e1248327.<链接> |
|
[37] |
Burridge P W, Diecke S, Matsa E, et al. Modeling cardiovascular diseases with patient-specific human pluripotent stem cell-derived cardiomyocytes[J]. Patient-Specific Induced Pluripotent Stem Cell Models: Generation and Characterization, 2016: 119-130.<链接> |
|
[38] |
Mendonça M C P, Soares E S, de Jesus M B, et al. PEGylation of Reduced Graphene Oxide Induces Toxicity in Cells of the Blood–Brain Barrier: An in Vitro and in Vivo Study[J]. Molecular pharmaceutics, 2016, 13(11): 3913-3924.<链接> |
|
[39] |
Eldaim A, Hashimoto O, Ohtsuki H, et al. Expression of uncoupling protein 1 in bovine muscle cells[J]. Journal of animal science, 2016, 94(12): 5097-5104.<链接> |
|
[40] |
Zhen A, Rezek V, Youn C, et al. Stem-cell based engineered immunity against HIV infection in the humanized mouse model[J]. JoVE (Journal of Visualized Experiments), 2016 (113): e54048-e54048.<链接> |
|
[41] |
Bastian N A, Bayne R A, Hummitzsch K, et al. Regulation of fibrillins and modulators of TGFβ in fetal bovine and human ovaries[J]. Reproduction, 2016, 152(2): 127-137.<链接> |
|
[42] |
Eto K, Takayama N, Nakamura S, et al. Method for producing differentiated cells: U.S. Patent 9,200,254[P]. 2015-12-1. |
|
[43] |
Eto K, Takayama N, Nakamura S, et al. Novel Method for Producing Differentiated Cells: U.S. Patent Application 14/925,508[P]. 2015-10-28. |
|
[44] |
Yamashita J, Takeda M. Cd82-positive cardiac progenitor cells: U.S. Patent Application 15/308,147[P]. 2015-4-16. |
|
[45] |
Cai Y, Sugimoto C, Arainga M, et al. Preferential Destruction of Interstitial Macrophages over Alveolar Macrophages as a Cause of Pulmonary Disease in Simian Immunodeficiency Virus–Infected Rhesus Macaques[J]. The Journal of Immunology, 2015, 195(10): 4884-4891.<链接> |
|
[46] |
Kojima S I, Kaku M, Kawata T, et al. Cranial suture-like gap and bone regeneration after transplantation of cryopreserved MSCs by use of a programmed freezer with magnetic field in rats[J]. Cryobiology, 2015, 70(3): 262-268.<链接> |
|
[47] |
Egawa E Y, Kitamura N, Nakai R, et al. A DNA hybridization system for labeling of neural stem cells with SPIO nanoparticles for MRI monitoring post-transplantation[J]. Biomaterials, 2015, 54: 158-167.<链接> |
|
[48] |
Durruthy J D, Sebastiano V. Derivation of GMP-Compliant Integration-Free hiPSCs Using Modified mRNAs[J]. Stem Cells and Good Manufacturing Practices: Methods, Protocols, and Regulations, 2015: 31-42.<链接> |
|
[49] |
Sato Y, Sasaki T, Takahashi S, et al. Development of a highly reproducible system to evaluate inhibition of cytochrome P450 3A4 activity by natural medicines[J]. Journal of Pharmacy & Pharmaceutical Sciences, 2015, 18(4): 316-327.<链接> |
|
[50] |
Burridge P W, Holmström A, Wu J C. Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells[J]. Current protocols in human genetics, 2015: 21.3. 1-21.3. 15.<链接> |
|
[51] |
Käding N. Hypoxia Regulates Host Cell Metabolism and Thereby Enhancing Clamydia Pneumonia Growth[D]. Zentrale Hochschulbibliothek Lübeck, 2015.<链接> |
|
[52] |
Lu S. Calcium Dependent Regulatory Mechanism in Wolfram Syndrome: A Dissertation[J]. 2015. |
|
[53] |
Garvican E R, Cree S, Bull L, et al. Viability of equine mesenchymal stem cells during transport and implantation[J]. Stem cell research & therapy, 2014, 5(4): 1.<链接> |
|
[54] |
Deng X, Terunuma H, Nieda M. Method for producing nk cell-enriched blood preparation: U.S. Patent Application 14/508,745[P]. 2014-10-7. |
|
[55] |
Foster K, Chaddock J, Penn C, et al. Non-cytotoxic protein conjugates: U.S. Patent 8,778,634[P]. 2014-7-15. |
|
[56] |
Ramathal C Y, Dumuthy-Durruthy J, Pera R A R, et al. Generation of male germ cells: U.S. Patent Application 14/904,396[P]. 2014-7-10 |
|
[57] |
Ince T A. Assays, methods and kits for analyzing sensitivity and resistance to anti-cancer drugs, predicting a cancer patient's prognosis, and personalized treatment strategies: U.S. Patent Application 14/894,595[P]. 2014-6-4. |
|
[58] |
Nishio M, Saeki K. Differentiation of human pluripotent stem cells into highly functional classical brown adipocytes[J]. Methods Enzymol, 2014, 537: 177-197.<链接> |
|
[59] |
Durruthy-Durruthy J, Briggs S F, Awe J, et al. Rapid and efficient conversion of integration-free human induced pluripotent stem cells to GMP-grade culture conditions[J]. PloS one, 2014, 9(4): e94231.<链接> |
|
[60] |
Koido S, Homma S, Okamoto M, et al. Treatment with Chemotherapy and Dendritic Cells Pulsed with Multiple Wilms' Tumor 1 (WT1)–Specific MHC Class I/II–Restricted Epitopes for Pancreatic Cancer[J]. Clinical Cancer Research, 2014, 20(16): 4228-4239.<链接> |
|
[61] |
Patz Jr E F. Antibodies Expressed by Intratumoral B Cells as the Basis for a Diagnostic Test for Lung Cancer[R]. DUKE UNIV DURHAM NC, 2014.<链接> |
|
[62] |
Kaku M, Shimasue H, Ohtani J, et al. A case of tooth autotransplantation after long-term cryopreservation using a programmed freezer with a magnetic field[J]. The Angle Orthodontist, 2014, 85(3): 518-524.<链接> |
|
[63] |
Kaku M, Koseki H, Kojima S, et al. Cranial bone regeneration after cranioplasty using cryopreserved autogenous bone by a programmed freezer with a magnetic field in rats[J]. CryoLetters, 2014, 35(6): 451-461.<链接> |
|
[64] |
Koido S, Kinoshita S, Mogami T, et al. Immunological assessment of cryotherapy in breast cancer patients[J]. Anticancer research, 2014, 34(9): 4869-4876.<链接> |
|
[65] |
Sazuka S, Katsuno T, Nakagawa T, et al. Fibrocytes are involved in inflammation as well as fibrosis in the pathogenesis of Crohn's disease[J]. Digestive diseases and sciences, 2014, 59(4): 760-768.<链接> |
|
[66] |
Lin S L, Lee S Y, Lin Y C, et al. Evaluation of mechanical and histological properties of cryopreserved human premolars under short-term preservation: A preliminary study[J]. Journal of Dental Sciences, 2014, 9(3): 244-248.<链接> |
|
[67] |
Poole E, Reeves M, Sinclair J H. The use of primary human cells (fibroblasts, monocytes, and others) to assess human cytomegalovirus function[J]. Human Cytomegaloviruses: Methods and Protocols, 2014: 81-98.<链接> |
|
[68] |
Garvican E R, Dudhia J, Alves A L, et al. Mesenchymal stem cells modulate release of matrix proteins from tendon surfaces in vitro: a potential beneficial therapeutic effect[J]. Regenerative medicine, 2014, 9(3): 295-308.<链接> |
|
[69] |
Skinner J A, Zurawski S M, Sugimoto C, et al. Immunologic characterization of a rhesus macaque H1N1 challenge model for candidate influenza vaccine assessment[J]. Clinical and Vaccine Immunology, 2014: CVI. 00547-14.<链接> |
|
[70] |
Terunuma H, Deng X, Nieda M. Method for producing nk cell-enriched blood preparation: U.S. Patent Application 14/780,394[P]. 2013-3-27. |
|
[71] |
Cho M, Yamazaki T, Endo M, et al. Anti-Phospholipase D4 Antibody: U.S. Patent Application 14/375,266[P]. 2013-1-31. |
|
[72] |
Bhandari S. Radiological, clinical and laboratory based studies in the pathogenesis of desmoid tumours in familial adenomatous polyposis[J]. 2013. |
|
[73] |
Gonzàlez Juncà A. Study of molecular mechanisms implicated in the TGF-beta oncogenic effect in Glioma[J]. 2013. |
|
[74] |
Koseki H, Kaku M, Kawata T, et al. Cryopreservation of osteoblasts by use of a programmed freezer with a magnetic field[J]. CryoLetters, 2013, 34(1): 10-19.<链接> |
|
[75] |
Naito H, Yoshimura M, Mizuno T, et al. The advantages of three‐dimensional culture in a collagen hydrogel for stem cell differentiation[J]. Journal of Biomedical Materials Research Part A, 2013, 101(10): 2838-2845.<链接> |
|
[76] |
Stec M, Baran J, Szatanek R, et al. Properties of monocytes generated from haematopoietic CD34+ stem cells from bone marrow of colon cancer patients[J]. Cancer Immunology, Immunotherapy, 2013, 62(4): 705-713.<链接> |
|
[77] |
Müller L, Brighton L E, Carson J L, et al. Culturing of human nasal epithelial cells at the air liquid interface[J]. Journal of visualized experiments: JoVE, 2013 (80).<链接> |
|
[78] |
Kalaszczynska I, Ruminski S, Platek A E, et al. Substantial differences between human and ovine mesenchymal stem cells in response to osteogenic media: how to explain and how to manage?[J]. BioResearch open access, 2013, 2(5): 356-363.<链接> |
|
[79] |
Tamai Y, Hasegawa A, Takamori A, et al. Potential Contribution of a Novel Tax Epitope–Specific CD4+ T Cells to Graft-versus-Tax Effect in Adult T Cell Leukemia Patients after Allogeneic Hematopoietic Stem Cell Transplantation[J]. The Journal of Immunology, 2013, 190(8): 4382-4392.<链接> |
|
[80] |
Kasai K, Nakashima H, Liu F, et al. Toxicology and biodistribution studies for MGH2. 1, an oncolytic virus that expresses two prodrug-activating genes, in combination with prodrugs[J]. Molecular Therapy-Nucleic Acids, 2013, 2: e113.<链接> |
|
[81] |
Deng X, Terunuma H, Nieda M. Method for producing nk cell-enriched blood preparation: U.S. Patent Application 13/980,777[P]. 2012-1-17. |
|
[82] |
Somm E, Bonnet N, Martinez A, et al. A botulinum toxin–derived targeted secretion inhibitor downregulates the GH/IGF1 axis[J]. The Journal of clinical investigation, 2012, 122(9): 3295.<链接> |
|
[83] |
Takaoka E, Sonobe H, Akimaru K, et al. Multiple sites of highly amplified DNA sequences detected by molecular cytogenetic analysis in HS-RMS-2, a new pleomorphic rhabdomyosarcoma cell line[J]. American journal of cancer research, 2012, 2(2): 141.<链接> |
|
[84] |
Fahlbusch F B, Dawood Y, Hartner A, et al. Cullin 7 and Fbxw 8 expression in trophoblastic cells is regulated via oxygen tension: implications for intrauterine growth restriction?[J]. The Journal of Maternal-Fetal & Neonatal Medicine, 2012, 25(11): 2209-2215.<链接> |
|
[85] |
Aloé S, Weber F, Behr B, et al. Modulatory effects of bovine seminal plasma on uterine inflammatory processes[J]. Reproduction in domestic animals, 2012, 47(1): 12-19.<链接> |
|
[86] |
Gupta A, Bhakta S. An integrated surrogate model for screening of drugs against Mycobacterium tuberculosis[J]. Journal of antimicrobial chemotherapy, 2012, 67(6): 1380-1391.<链接> |
|
[87] |
Saeki K. Feeder-Free Culture for High Efficiency Production of Subculturable Vascular Endothelial Cells from Human Embryonic Stem Cells[J]. Human Embryonic and Induced Pluripotent Stem Cells: Lineage-Specific Differentiation Protocols, 2012: 277-294.<链接> |
|
[88] |
Yamazaki T, Okabe H, Kobayashi S, et al. Cancer stem cell mass and process for production thereof: U.S. Patent Application 13/878,181[P]. 2011-10-6. |
|
[89] |
Deng X, Terunuma H, Nieda M. Method for producing nk cell-enriched blood product: U.S. Patent Application 13/577,476[P]. 2011-2-4. |
|
[90] |
Sugii S, Kida Y, Berggren W T, et al. Feeder-independent ips cell derivation from human and mouse adipose stem cells[J]. Nature protocols, 2011, 6(3): 346.<链接> |
|
[91] |
Shinada T, Akimoto T, Zhu Y, et al. Modulation of viability of live cells by focused ion‐beam exposure[J]. Biotechnology and bioengineering, 2011, 108(1): 222-225.<链接> |
|
[92] |
Huang M S, Chang W J, Huang H M, et al. Effects of transportation time after extraction on the magnetic cryopreservation of pulp cells of rat dental pulp[J]. Journal of Dental Sciences, 2011, 6(1): 48-52.<链接> |
|
[93] |
Sato D, Suzuki Y, Kano T, et al. Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients[J]. Nephrology Dialysis Transplantation, 2011, 27(3): 1090-1097.<链接> |
|
[94] |
Kamada H, Kaku M, Kawata T, et al. In-vitro and in-vivo study of periodontal ligament cryopreserved with a magnetic field[J]. American Journal of Orthodontics and Dentofacial Orthopedics, 2011, 140(6): 799-805.<链接> |
|
[95] |
Bui H T, Wakayama S, Mizutani E, et al. Essential role of paternal chromatin in the regulation of transcriptional activity during mouse preimplantation development[J]. Reproduction, 2011, 141(1): 67-77.<链接> |
|
[96] |
Takata Y, Kishine H, Sone T, et al. Generation of iPS cells using a BacMam multigene expression system[J]. Cell structure and function, 2011, 36(2): 209-222.<链接> |
|
[97] |
Benko Z, Zhao R Y. Zeocin for selection of bleMX6 resistance in fission yeast[J]. Biotechniques, 2011, 51(1): 57-60.<链接> |
|
[98] |
Abedini S, Kaku M, Kawata T, et al. Effects of cryopreservation with a newly-developed magnetic field programmed freezer on periodontal ligament cells and pulp tissues[J]. Cryobiology, 2011, 62(3): 181-187.<链接> |
|
[99] |
Oshima-Sudo N, Li Q, Hoshino Y, et al. Optimized method for culturing outgrowth endothelial progenitor cells[J]. Inflammation and Regeneration, 2011, 31(2): 219-227.<链接> |
|
[100] |
Araki N. Bioassay method for antibody against thyroid-stimulating hormone receptor, measurement kit for the antibody, and novel genetically modified cell for use in the bioassay method or the measurement kit: U.S. Patent Application 13/381,402[P]. 2010-6-24. |
|
[101] |
Foster K, Chaddock J, Marks P, et al. Fusion proteins: U.S. Patent 7,659,092[P]. 2010-2-9. |
|
[102] |
Mieno S, Boodhwani M, Robich M P, et al. Effects of diabetes mellitus on VEGF‐induced proliferation response in bone marrow derived endothelial progenitor cells[J]. Journal of cardiac surgery, 2010, 25(5): 618-625.<链接> |
|
[103] |
Kaku M, Kamada H, Kawata T, et al. Cryopreservation of periodontal ligament cells with magnetic field for tooth banking[J]. Cryobiology, 2010, 61(1): 73-78.<链接> |
|
[104] |
Kawata T, Kaku M, Fujita T, et al. Water molecule movement by a magnetic field in freezing for tooth banking[J]. Biomedical Research, 2010, 21(4).<链接> |
|
[105] |
Lee S Y, Chiang P C, Tsai Y H, et al. Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells[J]. Journal of Endodontics, 2010, 36(8): 1336-1340.<链接> |
|
[106] |
Huang Y H, Yang J C, Wang C W, et al. Dental stem cells and tooth banking for regenerative medicine[J]. Journal of Experimental & Clinical Medicine, 2010, 2(3): 111-117.<链接> |
|
[107] |
Kwon H J, Enomoto T, Shimogawara M, et al. Benchmarks[J]. Biotechniques, 2010, 48: 460-462.<链接> |
|
[108] |
Shimizu Y, Takamori A, Utsunomiya A, et al. Impaired Tax‐specific T‐cell responses with insufficient control of HTLV‐1 in a subgroup of individuals at asymptomatic and smoldering stages[J]. Cancer science, 2009, 100(3): 481-489.<链接> |
|
[109] |
Park H S, Cho S G, Park M J, et al. Bone marrow T cells are superior to splenic T cells to induce chimeric conversion after non-myeloablative bone marrow transplantation[J]. The Korean journal of internal medicine, 2009, 24(3): 252.<链接> |
|
[110] |
Enosawa S, Miyamoto Y, Ikeya T. Frozen cell immobilized product, primary hepatocyte culture tool, and method for producing primary hepatocyte culture tool: U.S. Patent Application 12/738,809[P]. 2008-9-11. |
|
[111] |
DePinho R A, Stommel J M. Receptor tyrosine kinase profiling: U.S. Patent Application 12/450,820[P]. 2008-4-11. |
|
[112] |
Mieno S, Clements R T, Boodhwani M, et al. Characteristics and Function of Cryopreserved Bone Marrow–Derived Endothelial Progenitor Cells[J]. The Annals of thoracic surgery, 2008, 85(4): 1361-1366.<链接> |
|
[113] |
Warren C. The Response of HN4 Cells to Porphyromonas gingivalis DNA[D]. , 2008. |
|
[114] |
Hikichi T, Wakayama S, Mizutani E, et al. Differentiation potential of parthenogenetic embryonic stem cells is improved by nuclear transfer[J]. Stem Cells, 2007, 25(1): 46-53.<链接> |
|
[115] |
Zaidi S K, Pande S, Pratap J, et al. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential[J]. Proceedings of the National Academy of Sciences, 2007, 104(50): 19861-19866.<链接> |
|
[116] |
Hikichi T, Wakayama S, Mizutani E, et al. Differentiation potential of parthenogenetic embryonic stem cells is improved by nuclear transfer[J]. Stem Cells, 2007, 25(1): 46-53.<链接> |
|
[117] |
Liu D G, Kobayashi T, Onishi A, et al. Relation between human decay‐accelerating factor (hDAF) expression in pig cells and inhibition of human serum anti‐pig cytotoxicity: value of highly expressed hDAF for xenotransplantation[J]. Xenotransplantation, 2007, 14(1): 67-73.<链接> |
|
[118] |
Ishii H, Iinuma A, Osumi K, et al. Canine tumor treatment method, pharmaceutical formulation applied thereto, and method of cryogenically preserving cells used therewith: U.S. Patent Application 11/465,892[P]. 2006-8-21. |
|
[119] |
Hatoya S, Sugiyama Y, Torii R, et al. Effect of co-culturing with embryonic fibroblasts on IVM, IVF and IVC of canine oocytes[J]. Theriogenology, 2006, 66(5): 1083-1090.<链接> |
|
[120] |
Sasaki M, Kato Y, Yamada H, et al. Development of a novel serum‐free freezing medium for mammalian cells using the silk protein sericin[J]. Biotechnology and applied biochemistry, 2005, 42(2): 183-188.<链接> |
|
[121] |
Haynes J E. Pseudonyms of Authors: Including Anonyms and Initialisms[M]. JE Haynes, 1882.<链接> |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 |
| 302-14681 | BAMBANKER BAMBANKER冻存液 |
120 mL | – |
| 306-14684 | BAMBANKER BAMBANKER冻存液 |
20 mLx5 | – |
| 306-95921 | BAMBANKER Direct BAMBANKER直接冻存液 |
20 mL | – |